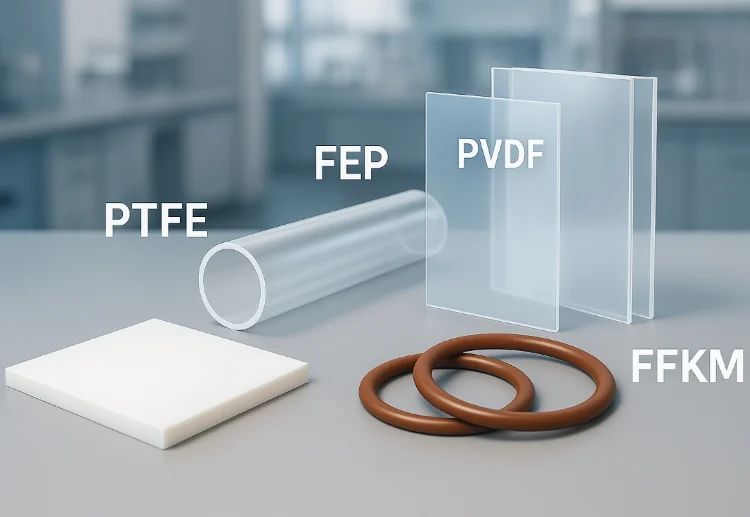
Fluoropolymers are high-performance materials synthesized by the homopolymerization or copolymerization of fluorine-containing monomers. Thanks to the exceptionally strong carbon–fluorine (C–F) bonds in their molecular structure, these materials exhibit unique and superior properties compared to conventional polymers.
The high bond energy of the C–F bond imparts outstanding thermal and chemical stability to the polymer backbone, resulting in excellent weather resistance. Additionally, the small atomic radius and low polarizability of fluorine contribute to distinctive surface characteristics—such as non-stick behavior, low friction, water repellency, and corrosion resistance—as well as remarkable electrical and optical performance, including high insulation, a low dielectric constant, and high light transmittance.
Fluoropolymers are generally categorized into fluororesins, fluoroelastomers (fluororubbers), and other specialty fluoropolymers. PTFE, PVDF, and FEP are the most widely used fluororesins, accounting for over 90% of the global market. Common types of fluoroelastomers include FKM, FEPM, FFKM, and others. Additional specialty fluoropolymers include fluorinated polyimides, polyurethanes, polyesters, epoxies, and perfluoropolyethers.
This article provides an in-depth overview of more than 30 common fluoropolymers, highlighting their properties, structures, and real-world applications across industries like aerospace, electronics, automotive, and chemical processing.
PTFE (Polytetrafluoroethylene)
PTFE, widely known under trade names such as “Teflon” and “4F,” is often referred to as the “King of Plastics” due to its exceptional combination of chemical, thermal, and electrical properties.
PTFE is a crystalline polymer produced through free-radical polymerization of tetrafluoroethylene (TFE). It has a high melting point of 327°C and an extremely high melt viscosity—up to 1010 Pa·s at 380°C—making it difficult to process using conventional thermoplastic methods. The material has a density of 2.13–2.19 g/cm³.
It exhibits excellent chemical resistance, a low dielectric constant (2.1), and thermal stability across a wide range of temperatures and frequencies. PTFE remains mechanically stable from –196°C to 260°C, with high impact strength even at low temperatures. However, it has relatively low tensile strength, wear resistance, and creep resistance compared to other engineering plastics.
To overcome these limitations, PTFE is often modified with additives such as glass fibers, carbon, bronze, or graphite, which enhance its mechanical performance for specific applications.

PTFE Gasket
One of PTFE’s most notable features is its extremely low coefficient of friction—lower than almost any other solid material. It also has a very high Limiting Oxygen Index (LOI), up to 95%, meaning it is highly flame-retardant and does not support combustion.
Typical applications of PTFE include corrosion-resistant linings, chemical pipes and fittings, heat exchangers, seals, insulators, medical components, and high-performance powder coatings.
Fluorinated Polyimide (FPI)
Fluorinated polyimide (FPI) is a rigid, high-performance polymer featuring a highly regular structure with imide rings in its backbone. It is synthesized by reacting fluorinated dianhydrides with fluorinated diamines via melt or solution polycondensation, followed by imidization to form fluorinated polyamic acid (FPAA).
FPI retains the well-known attributes of traditional polyimide (PI)—including high tensile strength, heat resistance, dimensional stability, and bending durability—while also offering enhanced transparency, electrical insulation, and a low dielectric constant. These characteristics make FPI particularly well-suited for advanced electronic applications such as OLED displays, where high optical transmittance is critical. Common uses include cover films, touch screen panel (TSP) layers, and high-transmittance support films.
FPI can be classified in multiple ways:
- By chemical structure: Diphenyl ether type, homophenyl type, benzophenone type, and biphenyl type FPI
- By polymer family: Fluorinated polyetherimide (FPEI), fluorinated polyamideimide (FPAI)
- By fluorination degree: Perfluorinated PI vs. partially fluorinated PI
Driven by demand in high-end sectors such as flexible electronics and thermal management systems, the global market for FPI continues to grow. However, core technologies remain concentrated in Japan and the United States, with Japan accounting for approximately 90% of global production.
While China has achieved large-scale production of some lower-barrier monomers like biphenyltetracarboxylic acid dianhydride (BPDA) and pyromellitic dianhydride (PMDA), specialty monomers such as hexafluorodianhydride (6FDA) are only recently seeing breakthroughs in domestic manufacturing, reducing reliance on foreign sources.
Chlorotrifluoroethylene–Vinyl Ether Copolymer (FEVE)
To overcome the limitations of PVDF in coating applications, researchers in Japan and the United States developed fluorocarbon resins containing hydroxyl functional groups. In 1982, Asahi Glass of Japan introduced FEVE, a copolymer of fluoroolefins and vinyl ether, under the trade name Lumiflon.
FEVE is an alternating copolymer composed of vinyl fluoride monomers and vinyl ether (or ester) monomers. The vinyl fluoride units form a protective structure around the vinyl ether segments, enhancing durability. Hydroxyl and carboxyl groups in the vinyl ether units allow FEVE to crosslink with isocyanates, enabling conventional curing processes without the need for high-temperature sintering.
Because of this, FEVE is soluble in esters, ketones, and aromatic solvents and can be applied using standard coating methods. It can form:
- Single-component, medium-temperature bake coatings using blocked polyisocyanates or melamine resins
- Two-component, room-temperature cure coatings when combined with polyisocyanates (e.g., HDI biuret or HDI trimer)
These fluorinated polyurethane coatings offer exceptional weather resistance, chemical resistance (acids, alkalis, solvents), and long-term gloss retention, making them ideal for high-performance architectural finishes and heavy-duty anti-corrosion coatings.
Fluorinated Ethylene Propylene (FEP)
FEP is a melt-processable fluoropolymer formed by copolymerizing tetrafluoroethylene (TFE) and hexafluoropropylene (HFP). It is a soft, crystalline plastic with a melting point of 304°C and a density of 2.15 g/cm³.
While FEP has lower tensile strength, wear resistance, and creep resistance compared to many engineering plastics, it offers excellent chemical inertness and thermal stability. Its dielectric constant remains low (2.1) across a broad range of temperatures and frequencies. It is non-flammable, with a limiting oxygen index (LOI) of up to 95%, and maintains performance from cryogenic conditions up to 392°C.

FEP Tube
FEP is available in granules for extrusion and molding, in powder form for fluidized bed or electrostatic coating, and as an aqueous dispersion. Semi-finished products include films, rods, sheets, and monofilaments.
Key applications of FEP include:
- Linings for pipes, valves, and chemical processing equipment
- Surface coatings for rollers and release sheets
- Wiring and cabling—e.g., aircraft hook-up wires, booster cables, alarm systems, oil well logging cables, and flat ribbon cables
- Solar energy—FEP film is used as a coating in solar collectors
Polychlorotrifluoroethylene (PCTFE)
PCTFE is a high-performance thermoplastic fluoropolymer synthesized via free-radical polymerization of chlorotrifluoroethylene (CTFE). It features a linear chain structure with repeating –CF2–CClF– units. Originally developed by IG Farben in Germany in the 1930s, PCTFE gained prominence during the Manhattan Project as a key material for uranium isotope separation. It was commercialized in 1949 under the name “Kel-F” by 3M in the U.S.

PCTFE Tube
PCTFE has excellent chemical resistance, thermal stability, low moisture absorption, and superior gas barrier properties. The fluorine atoms in the molecular structure provide inertness, while the presence of chlorine improves mechanical strength, hardness, and dimensional stability.
Although its chemical resistance and heat stability are slightly lower than PTFE and FEP due to the C–Cl bonds, PCTFE outperforms them in terms of rigidity, creep resistance, and impermeability. It remains stable in most aggressive environments, only breaking down in contact with molten alkali metals or strong oxidizing acids at high temperatures.
Key properties of PCTFE:
- Melting point: ~210°C
- Usable temperature range: –100°C to 150°C
- High dimensional precision and optical clarity
- Extremely low water vapor transmission rate
Common applications include:
- Vacuum system seals and gaskets
- Transparent pipes and sight gauges
- Electrical insulation parts
- Pharmaceutical and medical devices
- Aerospace and nuclear components
Polyvinylidene Fluoride (PVDF)
PVDF is a semi-crystalline fluoropolymer derived from the polymerization of vinylidene fluoride (VDF) or its copolymerization with small amounts of other fluorinated monomers. With a fluorine content of approximately 60%, PVDF offers an outstanding balance of chemical, mechanical, and electrical properties.
This high-performance material exhibits exceptional resistance to chemicals, UV radiation, weathering, and oxidation. It also provides excellent tensile strength, impact resistance, hardness, and wear resistance. The operating temperature range for PVDF typically spans –60°C to 150°C, making it suitable for both structural and chemical applications.
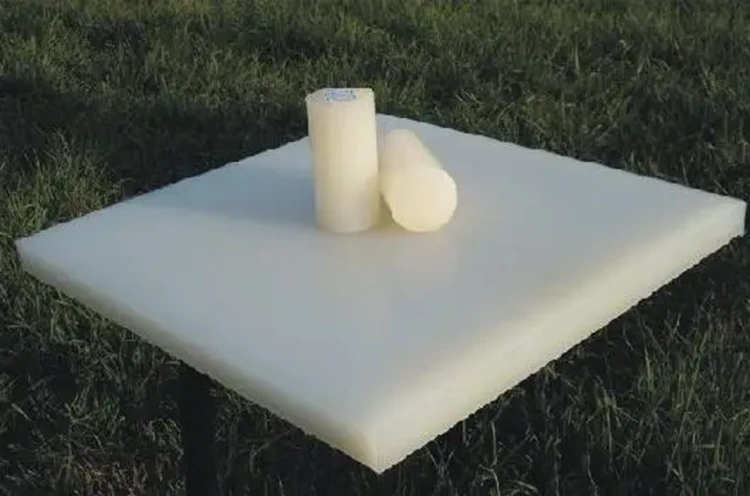
PVDF Sheet
Key properties of PVDF:
- High purity and excellent chemical resistance
- Superior fatigue and creep resistance
- Good flame retardancy and insulation performance
- Excellent processability via injection molding, extrusion, and welding
Main application areas:
- Petrochemical industry: Lining material for pipes, valves, tanks, and heat exchangers
- Electronics: High-purity chemical transport systems in semiconductor manufacturing
- Coatings: Used in high-performance fluorocarbon paints for architecture
- Energy storage: Battery binders, separators, gels, and adhesives in lithium-ion batteries—a rapidly growing market for PVDF
Fusible Polytetrafluoroethylene (PFA)
PFA, short for perfluoroalkoxy alkane, is a fully fluorinated fluoroplastic that retains all the exceptional properties of PTFE—such as chemical inertness, non-stick surface, and thermal stability—while also being melt-processable. This makes PFA an ideal alternative to PTFE for applications requiring complex shapes and precision molding.
PFA is produced by copolymerizing tetrafluoroethylene (TFE) with perfluoroalkyl vinyl ethers. This copolymer structure reduces melt viscosity and improves melt adhesion without sacrificing the high-performance characteristics associated with PTFE.

PFA Tube
Key properties of PFA:
- Continuous service temperature: –80°C to 260°C
- Outstanding resistance to virtually all chemicals
- Low coefficient of friction and excellent anti-stick behavior
- Stable electrical insulation properties across temperatures
- High tensile strength with 100–300% elongation
- Superior radiation resistance and flame retardancy
- Biocompatibility—safe for medical implants and devices
Common applications:
- Seals, gaskets, and valve linings in chemical processing
- Medical tubing and device components
- High-temperature wiring and cable insulation
- Non-stick and corrosion-resistant coatings
- Pump bushings, fittings, and reaction vessels
Ethylene Tetrafluoroethylene (ETFE)
ETFE is a tough, semi-crystalline fluoropolymer formed by copolymerizing ethylene and tetrafluoroethylene. Sometimes referred to as F40, ETFE is known as the most durable of the fluoroplastics—combining excellent chemical resistance and thermal stability with enhanced mechanical strength and radiation resistance.
Compared to PTFE, ETFE offers nearly twice the tensile strength (up to 50 MPa) and better adhesion to metal surfaces, enabling reliable tight-lining processes in corrosion-resistant piping systems. It maintains performance across a wide temperature range and is highly resistant to UV radiation and weathering.

ETFE Membrane in Modern Architecture
Key advantages of ETFE:
- Excellent mechanical toughness and flexibility
- High transparency and UV stability
- Outstanding impact resistance and abrasion resistance
- Processing temperature up to 300°C; service temperature up to 150°C
- High dielectric strength and chemical inertness
Main application areas:
- Architecture: Lightweight, translucent roofing and façade membranes (ETFE weighs just 1% of glass)
- Chemical industry: Linings for pipes, valves, and tanks
- Aerospace: Radiation-resistant films and insulation
- Electronics: Wire insulation and protective sheathing
ETFE films are highly ductile (elongation > 400%) and do not spontaneously ignite, making them ideal for modern structures that require durability, light transmission, and environmental resistance.
Tetrafluoroethylene–Hexafluoropropylene–Vinylidene Fluoride Copolymer (THV)
THV is a flexible, transparent fluoroplastic copolymer made from tetrafluoroethylene (TFE), hexafluoropropylene (HFP), and vinylidene fluoride (VDF). It combines the chemical resistance and non-flammability of traditional fluoroplastics with excellent processability—making it one of the most versatile fluoropolymers developed to date.
Unlike PTFE, which cannot be melt-processed, THV has a wide processing window and low melting point, allowing it to be extruded, co-extruded, injection-molded, blow-molded, laminated, dip-coated, and used in film applications. It is the softest commercial fluoroplastic, known for its superior flexibility and transparency.
Key properties of THV:
- Excellent chemical resistance against corrosive gases and liquids
- UV transparency and low refractive index—ideal for optical applications
- Low melting temperature, enabling co-processing with non-fluorinated polymers
- Good adhesion to metals and plastics—no surface treatment needed
- Radiation crosslinkable to improve high-temperature resistance and strength
Common applications of THV include:
- Multilayer fuel lines and chemical hoses
- Flexible optical fibers and light control materials
- Architectural films and solar panel encapsulation
- Protective coatings and clear tubing for aggressive environments
- Specialized containers, molded parts, and pressure-resistant linings
THV’s transparency across the UV to IR spectrum, combined with chemical inertness and bondability, makes it a strong candidate for emerging applications in solar, optics, and precision electronics.
Ethylene Chlorotrifluoroethylene (ECTFE)
ECTFE is a tough, semi-crystalline copolymer made from ethylene and chlorotrifluoroethylene (CTFE). It offers outstanding corrosion resistance and low permeability, making it an ideal choice for demanding chemical processing environments. Among all fluoropolymers, ECTFE is particularly notable for its resistance to strong oxidizers, chlorine, and a broad range of inorganic and organic chemicals.
ECTFE maintains performance over a wide temperature range—from cryogenic conditions up to 149°C. It also provides excellent mechanical properties, impact resistance, and electrical insulation, even in aggressive service environments.
Key properties of ECTFE:
- Exceptional resistance to acids, bases, solvents, and chlorine-based compounds
- Very low water absorption and permeation rate
- High surface smoothness, which resists microbial growth
- Good mechanical toughness and impact strength
- Stable dielectric performance over a wide frequency range
An interesting comparison by Ausimont (now part of Solvay) showed that, under 1000x magnification, ECTFE’s surface (specifically HALAR®-lined materials) remained smoother and had significantly fewer microbial adhesion sites than surfaces made of polypropylene (PP), PVDF, or PVC. This gives ECTFE a distinct hygienic advantage in chemical and pharmaceutical applications.
Main applications of ECTFE:
- Drainage and wastewater systems in chemical and petrochemical plants
- Scrubbers, exhaust ducts, and chemical cleaning systems
- Lining material for tanks, vessels, and pipelines handling aggressive media
- Wire and cable insulation in harsh industrial environments
Polyvinyl Fluoride (PVF)
Polyvinyl fluoride (PVF) is a partially crystalline fluoropolymer produced by the homopolymerization of vinyl fluoride. With the lowest fluorine content among commercial fluoroplastics, PVF offers a unique balance of cost-effectiveness, transparency, and durability—making it ideal for thin film applications.
PVF is a white, powdery thermoplastic with a melting point of approximately 190–200°C and a decomposition temperature above 210°C. It has a usable temperature range from –100°C to 150°C and a molecular weight typically ranging from 60,000 to 180,000.

PVF Film for Photovoltaic Backsheet
Key properties of PVF:
- High electrical insulation and transparency (including UV transmittance)
- Good weather resistance, chemical resistance, and aging performance
- Strong toughness and flexibility in thin film form
- Low cost relative to other fluoroplastics
Main applications of PVF:
- Backsheets for photovoltaic modules and solar panels
- Protective films for architectural panels and aircraft interiors
- Packaging for corrosive substances and oils
- Agricultural films and electrical insulation materials
PVF is primarily used in film form, which significantly enhances its barrier properties against UV, chemicals, moisture, and environmental degradation—making it indispensable in long-life outdoor applications.
Fluorinated Polyurethane
Polyurethane (PU) is a highly versatile polymer composed of repeating carbamate (urethane) groups. It is widely used in applications ranging from foams, elastomers, and adhesives to coatings and synthetic fibers. While polyurethane offers excellent mechanical properties such as strength, elasticity, and hardness, it generally has poor resistance to water, weathering, and chemicals.
To overcome these limitations, fluorine atoms can be introduced into the polyurethane molecular structure. Fluorination enhances the polymer’s surface and thermal characteristics while preserving its inherent toughness and elasticity.
Benefits of fluorinated polyurethane include:
- Lower surface energy for enhanced water and oil repellency
- Improved heat resistance and oxidation stability
- Reduced dielectric constant and refractive index
- Enhanced chemical resistance and anti-fouling performance
- Superior weatherability and flame retardancy
The incorporation of C–F bonds results in higher bond energy and a more chemically inert surface, making fluorinated polyurethanes suitable for demanding environments. These materials are increasingly used in:
- Weather-resistant and anti-corrosion coatings
- Low-dielectric insulation materials for microelectronics
- Aerospace and military-grade composites
- Biomedical applications such as implant coatings and medical tubing
- Protective coatings for cultural heritage preservation
Fluorinated polyurethane represents an advanced class of materials that combines the flexibility of PU with the chemical resilience of fluoropolymers, offering multifunctional performance for both industrial and specialized applications.
Fluororubber (Fluoroelastomer)
Fluororubber—also known as fluoroelastomer—is a synthetic elastomer containing fluorine atoms on its polymer backbone or side chains. It is well-known for its exceptional resistance to heat, oil, fuel, and aggressive chemicals, while still offering strong mechanical properties and elasticity. Due to this rare combination of features, fluororubber is widely used in extreme sealing environments such as aerospace, automotive, chemical processing, and military industries.
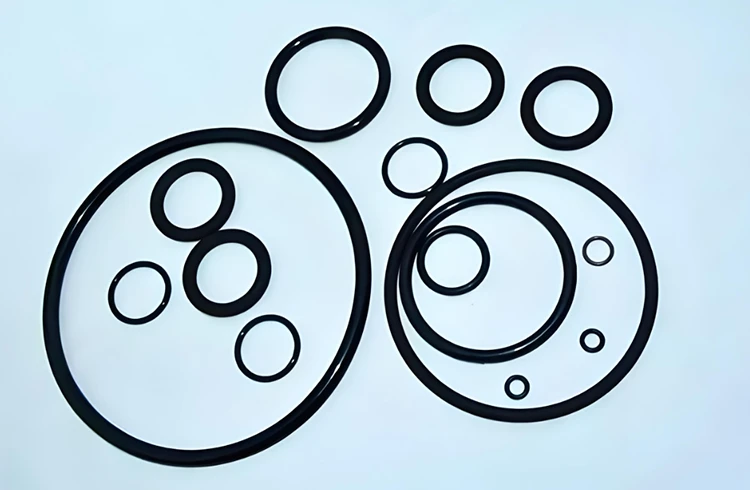
Perfluoroether Rubber (FFKM)
Main categories of fluororubber include:
- FKM: The most common type, made from vinylidene fluoride, hexafluoropropylene, and tetrafluoroethylene. Includes Type 26, Type 246, and perfluoroether rubber grades.
- FFKM (Perfluoroelastomer): Made from tetrafluoroethylene and perfluorovinyl ethers. Offers the highest chemical and temperature resistance (up to 325°C) and is often used in semiconductor, aerospace, and pharmaceutical applications.
- FEPM: A copolymer of tetrafluoroethylene and propylene. Resistant to acids, alkalis, steam, oils, and brake fluids. Used in automotive, chemical, and food industries.
- FZ (Fluorinated Phosphazene Rubber): Contains no carbon–carbon bonds in its backbone. Offers excellent resistance to ozone, low temperatures (down to –68°C), and chemical aging. Also shows high flame retardancy and flex resistance.
Key properties of fluororubbers:
- Excellent high-temperature performance: typically –20°C to 200°C; some grades up to 325°C
- Outstanding resistance to oils, fuels, and solvents
- Low gas permeability and strong mechanical strength
- Good aging resistance under heat, UV, and ozone
Common applications:
- O-rings, gaskets, seals, and diaphragms in engines and hydraulic systems
- Fuel system components in aerospace and automotive sectors
- High-performance hoses, valve seats, and bottle stoppers
- Extreme sealing solutions in semiconductor processing equipment
Fluororubber’s performance under extreme conditions makes it an essential material in industries where safety, durability, and chemical stability are critical. Among synthetic rubbers, it remains one of the most versatile and reliable sealing solutions.
Fluorinated Acrylate Polymer
Fluorinated acrylate polymers are specialized materials derived by incorporating fluorinated groups into conventional acrylate polymer chains. Acrylate polymers are widely used in coatings, textiles, paper finishing, and construction due to their ease of processing, strong film-forming ability, and cost efficiency. However, they often lack sufficient hydrophobicity, oleophobicity, and weather resistance—limitations that can be addressed through fluorination.
By introducing fluorine atoms—especially CF3 and CF2 groups—into the polymer chain, surface tension is reduced, improving resistance to water, oil, dirt, and chemicals. These polymers also show improved thermal and UV stability, making them ideal for outdoor and high-performance coatings.
Advantages of fluorinated acrylate polymers:
- Enhanced water- and oil-repellency
- Improved weatherability and anti-fouling properties
- Increased surface smoothness and self-cleaning effect
- Good chemical resistance and low surface energy
- Certain types offer antibacterial and bio-inert properties
Types of fluorinated acrylate polymers:
- Homopolymers: Offer strong repellency but are costly and brittle
- Copolymer blends: Fluorinated monomers copolymerized with standard acrylates or functional monomers to balance performance and cost
Common fluorinated acrylate monomers include:
- Hexafluorobutyl acrylate
- Dodecafluoroheptyl methacrylate
- Perfluorosulfonamide (meth)acrylates
- Perfluoroalkyl ethyl acrylates
Important note: While longer-chain perfluoroalkyl acrylates (C8 and above) show superior performance, they are environmentally persistent and harder to degrade. Many countries now regulate or restrict their use, shifting demand to shorter-chain (C6 and below) alternatives.
Key application areas:
- Textile and leather coatings for water and stain repellency
- Self-cleaning architectural and automotive coatings
- Non-stick paper, packaging, and release liners
- Protective films for electronics and solar panels
Fluorinated Polycarbonate
Polycarbonate (PC) is a high-performance thermoplastic known for its excellent impact resistance, optical clarity, and electrical insulation. The most common form—based on bisphenol A (BPA)—is widely used in automotive parts, lighting systems, electronics, building materials, and packaging. However, conventional PC can fall short in high-end applications requiring enhanced thermal, chemical, and dielectric properties.
Fluorinated polycarbonate addresses these limitations by incorporating fluorinated building blocks such as bisphenol AF (BPAF), which contains –CF3 groups. These groups introduce stronger intermolecular interactions and reduce the polymer’s polarizability, resulting in superior performance.
Benefits of fluorinated polycarbonate:
- Increased thermal stability and higher glass transition temperature (Tg)
- Lower dielectric constant for improved insulation
- Reduced water absorption and improved dimensional stability
- Enhanced chemical resistance and weatherability
- Improved transparency and lower refractive index
These advantages make fluorinated polycarbonates suitable for next-generation optical films, aerospace components, and microelectronic insulation materials. They are also promising candidates for applications in harsh environments where conventional PC would degrade more quickly.
Due to their complex synthesis and high cost, fluorinated polycarbonates are mainly used in specialized fields. However, their development is accelerating as the demand for lightweight, thermally stable, and low-dielectric materials increases in high-frequency electronic and optical applications.
P(VDF-co-CTFE) – Vinylidene Fluoride–Chlorotrifluoroethylene Copolymer
P(VDF-co-CTFE) is a fluorinated copolymer formed by polymerizing vinylidene fluoride (VDF) with chlorotrifluoroethylene (CTFE). Initially developed for military applications in the 1950s, it was commercialized under the brand name Kel® F by Kellogg in 1955.
By adjusting the ratio of VDF and CTFE, the copolymer’s properties—such as flexibility, crystallinity, and thermal performance—can be fine-tuned. Specifically, the presence of CTFE reduces crystallinity and increases amorphous regions, giving the material improved toughness and better processability compared to pure PVDF or PCTFE.
Key features of P(VDF-co-CTFE):
- Adjustable glass transition temperature (Tg) between that of PVDF (–40°C) and PCTFE (+45°C)
- Enhanced flexibility and elongation
- Good chemical and weather resistance
- Low moisture permeability
- Excellent adhesion to metals and other substrates
Typical applications include:
- Inner linings for fluid transport pipes in oil and gas systems (especially subsea and onshore pipelines)
- Protective layers for flexible tubing, diaphragms, and films
- Barrier materials in high-purity chemical environments
P(VDF-co-CTFE) is valued for its balance of flexibility, barrier performance, and chemical stability—making it well-suited for aggressive environments where traditional plastics would fail.
P(VDF-co-TrFE) – Vinylidene Fluoride–Trifluoroethylene Copolymer
P(VDF-co-TrFE) is a semi-crystalline fluorinated copolymer made by copolymerizing vinylidene fluoride (VDF) with trifluoroethylene (TrFE). It exhibits strong ferroelectric and piezoelectric behavior, making it a key material in sensors, actuators, and energy harvesting devices.
With a VDF molar content between 50% and 80%, P(VDF-co-TrFE) forms a β-phase crystalline structure that supports spontaneous electric polarization. After poling (aligning the dipoles through heat or an electric field), the material demonstrates high piezoelectric coefficients and electromechanical coupling.
Key properties of P(VDF-co-TrFE):
- High piezoelectric response (d31 and d33 up to ±25 pC/N)
- Good mechanical flexibility and stretchability
- Higher electromechanical coupling (kt) than ceramic counterparts
- Usable in thin film, fiber, and molded forms
- Thermoplastic behavior—allows easy processing compared to brittle piezoceramics
Main application areas:
- Piezoelectric sensors and transducers (pressure, vibration, strain)
- Ultrasound imaging and hydrophones
- Micro-speakers and microphones
- Energy harvesting systems and smart wearables
- Pyroelectric and electroactive memory devices
Compared to traditional piezoceramics like PZT, P(VDF-co-TrFE) offers better flexibility, processability, and compatibility with soft electronics—making it ideal for medical, consumer, and structural monitoring applications.
Polytrifluorostyrene (PTFS)
Polytrifluorostyrene (PTFS) is a homopolymer derived from trifluorostyrene (TFS), structurally similar to polytetrafluoroethylene (PTFE), but with one fluorine atom replaced by a phenyl (benzene) ring. As a result, PTFS exhibits distinct physical and chemical properties, making it a subject of growing interest in the field of fluorinated functional materials.
PTFS has a relatively high glass transition temperature (~210°C) and is amorphous with very low crystallinity. Unlike PTFE, it is brittle at room temperature and dissolves in several organic solvents—limiting its direct use in structural components but opening new opportunities for functional applications.
Key characteristics of PTFS:
- High thermal stability and glass transition temperature
- Solubility in a variety of polar organic solvents
- Low surface energy and chemical reactivity
- Brittle and non-wear-resistant without modification
To expand its functionality, PTFS can be chemically modified (functionalized) to introduce active groups for advanced applications:
- Sulfonation: Produces cation exchange membranes for fuel cells and ion separation
- Nitration: Enables the development of high-birefringence optical films
Functionalization methods:
- Pre-polymerization: Synthesizing modified trifluorostyrene monomers and then copolymerizing—more versatile but chemically complex
- Post-polymerization: Modifying PTFS directly after polymerization—simpler but limited in group diversity and risk of crosslinking
Although still in the research and development stage, PTFS has potential in advanced membrane technologies, optical films, and specialty coatings—especially where high fluorine content and solubility are advantageous.
Perfluorosulfonic Acid Resin
Perfluorosulfonic acid (PFSA) resin is a high-performance ionomer known for its extraordinary chemical stability, high proton conductivity, and thermal resistance. It is considered one of the strongest solid superacids and is a critical material in the manufacture of proton exchange membranes (PEMs) for fuel cells and ion exchange membranes for electrochemical processes.
PFSA resins are typically synthesized by copolymerizing tetrafluoroethylene (TFE) with perfluorovinyl ether monomers that contain sulfonic acid functional groups. The presence of highly electronegative fluorine atoms imparts exceptional resistance to chemical attack and oxidation, while the sulfonic acid groups provide strong ion conductivity.
Key properties of PFSA resin:
- Excellent thermal stability (stable up to ~200°C)
- Outstanding chemical resistance—even in strong acids and bases
- High proton conductivity due to sulfonic acid groups
- Good mechanical strength and processability as a thermoplastic
- Long-term durability under electrochemical conditions
To produce membranes, PFSA resin is typically melt-processed at 160–230°C and extruded into films. These films are then softened and laminated for mechanical reinforcement. Because of its unique structure, the resin combines the benefits of fluoropolymer backbones with ion-conducting functional groups.
Primary application areas:
- Fuel cells: Proton exchange membranes (PEMs) for hydrogen energy systems and electric vehicles
- Chlor-alkali industry: Ion exchange membranes for brine electrolysis
- Electrolyzers: Hydrogen production via water electrolysis
- Battery systems: Flow battery separators and proton-conducting films
- Environmental engineering: Acid recovery and heavy metal ion separation
With the global push for clean energy, the demand for PFSA resin is rapidly increasing—especially as a core material in hydrogen fuel cells and electrolysis systems used in new energy vehicles and energy storage technologies.
Fluorosilicone Rubber
Fluorosilicone rubber is a hybrid elastomer that combines the benefits of silicone and fluorinated compounds. It was developed to overcome the weaknesses of conventional silicone rubber—particularly its poor resistance to fuels, oils, and aggressive chemicals—while preserving its excellent flexibility, thermal stability, and weather resistance.
The backbone of fluorosilicone rubber is based on polysiloxane (silicon-oxygen chains), with some methyl groups replaced by trifluoropropyl side chains. This modification significantly enhances oil, fuel, and solvent resistance while retaining the key characteristics of traditional silicone materials.
Key advantages of fluorosilicone rubber:
- Excellent low-temperature flexibility (as low as –60°C)
- Outstanding resistance to fuels, oils, and hydraulic fluids
- Stable performance over a wide temperature range (–60°C to 200°C)
- Good resistance to ozone, UV, and weathering
- Low compression set and high resilience
Typical applications include:
- Sealing components in aerospace and automotive fuel systems
- Gaskets, O-rings, and hoses in harsh chemical environments
- Medical and industrial tubing exposed to solvents
- Electrical insulators requiring oil and heat resistance
Fluorosilicone rubber is especially useful in applications where exposure to fuels and chemicals is unavoidable, and where traditional silicone or fluorocarbon rubbers would degrade over time. Though more expensive than standard silicones, its performance in extreme environments justifies the cost in mission-critical applications.
Fluorinated Polyester
Fluorinated polyester is a class of modified polyesters in which some hydrogen atoms on the polymer backbone or side chains are replaced with fluorine atoms. This structural change enhances the material’s surface, thermal, and chemical properties—expanding its use in high-performance coatings, films, and fibers.
Based on fluorine placement, fluorinated polyesters are categorized into three types:
- Fluorine atoms in the main chain
- Fluorine atoms in the side chain
- Fluorine atoms in both the main and side chains
Benefits of fluorinated polyester include:
- Lower surface free energy for excellent water- and oil-repellency
- Reduced friction coefficient and dielectric constant
- Improved weatherability and oxidation resistance
- High transparency and light transmission in thin films
- Strong resistance to chemical attack and UV degradation
Application areas:
- Anti-fouling, weather-resistant coatings for buildings and infrastructure
- Waterproof and anti-fog films for electronics and optics
- Self-cleaning textiles and high-performance technical fibers
- Intermediate resins for producing fluorinated polyurethane coatings
Fluorinated polyesters are gaining attention in industries demanding materials with surface protection and environmental durability. Hydroxyl-terminated versions can also serve as prepolymers in advanced polyurethane systems for coatings and adhesives.
Fluorinated Epoxy Resin
Epoxy resins are widely used thermosetting polymers valued for their strong adhesion, chemical resistance, electrical insulation, and mechanical strength. However, conventional epoxy resins often fall short in hydrophobicity, oil resistance, and long-term weather stability. Fluorination modification addresses these shortcomings by introducing fluorinated groups into the resin backbone or side chains.
By incorporating fluorine atoms—particularly CF3 or CF2 units—the epoxy resin gains significantly enhanced performance due to the strong C–F bond (bond energy ~486 kJ/mol), low polarizability, and high electronegativity of fluorine.
Key improvements from fluorination:
- Excellent chemical resistance (acids, bases, solvents)
- Lower surface energy, resulting in better water and oil repellency
- Wider operating temperature range and better thermal stability
- Reduced dielectric constant and refractive index
- Improved aging resistance, UV resistance, and anti-fouling behavior
The fluorine atoms also arrange themselves helically around the polymer’s carbon backbone, forming a three-dimensional shield that protects the resin from environmental degradation and chemical attack.
Application fields include:
- High-performance coatings for aerospace, marine, and automotive use
- Electronic packaging and insulation for microelectronics
- Optical adhesives and anti-reflective coatings
- Specialty composites for defense and satellite technologies
Due to its cost and complexity, fluorinated epoxy resin is typically reserved for high-end applications where long-term reliability, electrical performance, and environmental resistance are critical. As demand grows for next-generation materials, its use is expected to expand further into precision electronics and advanced infrastructure.
Fluorinated Polyetheretherketoneketone (PEEKK)
Polyetheretherketoneketone (PEEKK) is a high-performance thermoplastic polymer in the PAEK family, structurally similar to PEEK but with additional ketone groups that enhance rigidity and thermal stability. It offers excellent mechanical properties, chemical resistance, radiation tolerance, and electrical insulation—making it suitable for aerospace, nuclear, and electronics applications.
However, like many high-performance thermoplastics, unmodified PEEKK suffers from high processing temperatures and poor solubility. Introducing fluorine atoms into the polymer backbone or side chains is an effective way to address these limitations.
Benefits of fluorinated PEEKK:
- Improved thermal stability and flame retardancy
- Lower dielectric constant and reduced refractive index
- Enhanced solubility in organic solvents—easier processing
- Lower moisture absorption for dimensional stability
- Increased optical transparency and light transmission efficiency
The fluorine atoms decrease intermolecular interactions and reduce the polarizability of the polymer, which improves flexibility and optical properties while maintaining high-performance mechanical integrity.
Application potential:
- Low-dielectric materials for high-speed electronics and 5G devices
- Optical waveguide components and transparent structural films
- Gas-selective membranes for environmental and medical use
- Radiation-resistant insulators in aerospace and nuclear environments
Fluorinated PEEKK is a promising next-generation material that blends exceptional strength with processability and advanced dielectric behavior, making it a competitive candidate in high-tech and mission-critical fields.
Fluorinated Polyarylether
Fluorinated polyarylethers are a class of high-performance polymers formed by introducing fluorine atoms into the backbone or side chains of traditional polyarylethers. This structural modification enhances thermal, electrical, and surface properties, making them suitable for use in advanced electronics, optical systems, and chemical-resistant applications.
Polyarylethers are already known for their thermal stability, mechanical strength, and low moisture absorption. Adding fluorinated groups—such as trifluoromethyl (–CF3) or hexafluoroisopropyl (–C(CF3)2)—further improves performance by reducing intermolecular interactions and enhancing dielectric properties.
Key performance enhancements from fluorination:
- Lower dielectric constant and dissipation factor
- Improved flame retardancy and thermal stability
- Reduced moisture absorption and better hydrolytic stability
- Higher solubility in organic solvents—easier processing
- Improved transparency and color stability
Common fluorinated monomers used:
- Hexafluorobisphenol A (6F-BPA) or its derivatives
- Fluorinated diphenyl ether and biphenyl units
Application areas:
- Insulating materials for ultra-large-scale integrated circuits (ULSI)
- Low-dielectric films for high-speed electronics
- Gas separation membranes and filtration systems
- Optical waveguides and photonic devices
Due to their balanced mechanical properties, dimensional stability, and excellent electrical insulation, fluorinated polyarylethers are gaining traction in microelectronics, telecommunications, and clean energy systems where reliability and signal integrity are paramount.
Fluorinated Poly(arylether nitrile) (FPEN)
Poly(arylether nitrile) (PEN) is a high-performance engineering plastic known for its excellent heat resistance, mechanical strength, dielectric stability, and radiation resistance. It is widely used in aerospace, electronics, and biomedical applications. However, traditional PEN materials have limited solubility and processability, which can be improved through fluorination.
Fluorinated PEN (FPEN) is synthesized by introducing fluorine-containing units and phenolphthalein structures into the polymer backbone. This modification significantly enhances solubility and fine-tunes the thermal and dielectric properties of the polymer.
Performance enhancements of FPEN:
- Improved solubility in solvents such as DMAc, DMF, chloroform, and butanone
- Higher thermal stability, with glass transition temperatures (Tg) typically exceeding 200°C
- Reduced dielectric constant—ideal for electronic insulation
- Enhanced flexibility and film-forming ability
The addition of fluorine reduces intermolecular forces and polarizability, enabling easier film formation and better compatibility with composite materials or coatings. It also provides hydrophobicity and better dimensional stability under harsh environmental conditions.
Application areas of FPEN:
- Flexible circuit substrates and insulating films
- Microwave and RF electronic components
- Thermally stable coatings and laminates
- Radiation-resistant materials in aerospace and nuclear systems
With growing demand for high-frequency and high-temperature-resistant polymers, FPEN is positioned as a strong candidate for advanced flexible electronics, precision sensors, and next-generation power systems.
Amorphous Fluoropolymer
Amorphous fluoropolymers are a unique subset of fluorinated materials developed in the late 1980s to meet the need for transparent, soluble, and optically advanced fluoropolymers. Unlike semi-crystalline fluoroplastics such as PTFE or FEP, amorphous fluoropolymers lack ordered crystalline domains—resulting in high transparency, isotropic mechanical behavior, and excellent optical properties.
The most widely known amorphous fluoropolymer is the copolymer of perfluoro-2,2-dimethyl-1,3-dioxole (PDD) and tetrafluoroethylene (TFE), commercialized as Teflon® AF by DuPont. It combines the thermal and chemical resistance of conventional fluoropolymers with unmatched transparency and low refractive index.
Key characteristics of amorphous fluoropolymers:
- High light transmittance across the UV–IR spectrum
- Low refractive index (~1.29)—ideal for optical applications
- Excellent dielectric properties and low dissipation factor
- Solubility in specialty fluorinated solvents
- High gas permeability and flexible processability
Common applications:
- Optical fibers, lenses, and light-guiding films
- Gas separation membranes
- Analytical and diagnostic instruments
- Medical devices and transparent tubing
- High-frequency electronic substrates and waveguides
Thanks to their amorphous structure, these fluoropolymers provide superior clarity and processing versatility without compromising on chemical inertness. Their performance is particularly valued in semiconductor manufacturing, photonics, and high-precision electronics where light transmission and environmental resistance are critical.
Perfluoropolyether (PFPE)
Perfluoropolyether (PFPE) is a class of fully fluorinated, low-molecular-weight polymers known for their exceptional chemical stability, low surface energy, and broad liquid temperature range. Typically existing as clear, colorless liquids at room temperature, PFPEs are widely used as lubricants, especially in aerospace, semiconductor, and vacuum applications where conventional oils fail.

Perfluoropolyether
PFPE molecules are composed only of carbon (C), fluorine (F), and oxygen (O), making them chemically inert and thermally stable. Their performance does not degrade in high-vacuum, oxidizing, or corrosive environments.
Key characteristics of PFPE:
- Wide liquid temperature range (–90°C to +250°C)
- Extremely low vapor pressure—ideal for high-vacuum systems
- High thermal and oxidative stability
- Excellent lubricity and anti-wear performance
- Non-flammable, non-reactive, and compatible with most metals and elastomers
PFPE types and production methods:
- K-type and D-type PFPEs: Produced via anionic polymerization
- D-type: Synthesized from tetrafluorooxetane via ring-opening polymerization and fluorination
- K-type: Based on hexafluoropropylene oxide (HFPO) using fluoride ion catalysts
Application fields include:
- Aerospace: Space-grade greases, bearing lubricants, and cryogenic components
- Semiconductors: Vacuum pump lubricants for plasma etching, LPCVD, and ion implantation
- Industrial equipment: High-temperature conveyor chains, paper machines, and textile rollers
- Chemical processing: Sealing and lubricating in reactive gas environments
PFPEs are the lubricant of choice in situations where long-term reliability, extreme thermal range, and chemical inertness are essential. Their unmatched stability and compatibility make them irreplaceable in advanced technology sectors.
If you’re designing high-performance systems or sourcing advanced materials, understanding fluoropolymers is critical. From chemical resistance to thermal stability, the right polymer choice can make or break your application. Need help selecting the right fluoropolymer for your project? Contact our material specialists today to get expert recommendations tailored to your industry.