Introduction
When it comes to medical devices, precision and safety are non-negotiable. From the tiniest catheter to the tubing that delivers life-saving medication, every component must perform flawlessly under pressure. In this high-stakes environment, both the choice of material and the manufacturing method can make the difference between product success and failure.
That’s why I’ve seen silicone extrusion rise to the top as a preferred manufacturing process in the medical field. Not only is medical-grade silicone inherently biocompatible and flexible, but the extrusion method itself offers incredible consistency, tight tolerances, and scalability. Whether you’re developing a single-use device or a long-term implant, this combination of material and method ensures optimal performance across a wide range of applications.
In this article, I’ll walk you through the unique advantages of silicone extrusion in medical manufacturing. We’ll cover topics like biocompatibility, micro-precision, sterilization durability, and even cost efficiency—all backed by real-world use cases and production insights.
Let’s begin by understanding exactly how silicone extrusion works and why it’s become a cornerstone of modern medical innovation.
Overview of Silicone Extrusion
What is Silicone Extrusion?
![]()
Silicone extrusion is a continuous manufacturing process used to create long, uniform profiles such as tubes, cords, and custom shapes. The process starts with raw silicone—typically a high-consistency rubber (HCR) or liquid silicone rubber (LSR)—that is fed into an extruder. This material is then pushed through a die, forming it into a specific cross-sectional shape. Once shaped, the silicone is cured using heat, often through a high-temperature vulcanization (HTV) oven or inline curing system, depending on the formulation.
The roots of silicone extrusion trace back to the mid-20th century when the medical industry began adopting synthetic polymers for their unique properties. Over the decades, the process has evolved into a clean, tightly controlled method, capable of producing everything from millimeter-scale tubing for catheters to thick-walled profiles for sealing systems in diagnostic machines.
Common products produced through extrusion include:
- Tubing for drug delivery or fluid transfer
- Cords and gaskets used in respirators or implants
- Custom profiles for sealing or device integration
The strength of silicone extrusion lies in its repeatability, precision, and ability to run at scale—key factors in medical manufacturing where uniformity can directly affect patient safety.
Types of Silicone Used
In medical manufacturing, not all silicone is created equal. The choice of compound can significantly influence biocompatibility, clarity, flexibility, and cure behavior.
- Medical-Grade vs. Food-Grade Silicone:
Medical-grade silicone undergoes stricter testing for cytotoxicity, sensitization, and implant safety, often in accordance with USP Class VI or ISO 10993 standards. While food-grade silicones are safe for incidental contact, they typically don’t meet the same implantability and long-term exposure requirements. - Platinum-Cured vs. Peroxide-Cured:
Most medical extrusions use platinum-cured silicone, known for its purity, transparency, and low extractables. Peroxide-cured silicones, while cheaper, often release byproducts not suitable for critical healthcare applications. - Compliance and Certification:
Trusted suppliers ensure their silicone formulations are compliant with FDA 21 CFR 177.2600, USP Class VI, and ISO 10993 for use in medical environments. At Kinsoe, we source high-purity silicone blends that meet these global standards, ensuring compatibility with even the most sensitive medical procedures.
Why Silicone Extrusion Is Ideal for the Medical Industry
Biocompatibility and Safety
One of the most critical requirements for any material used in the medical industry is biocompatibility. Silicone stands out as one of the safest materials for contact with human tissue and fluids. It’s non-toxic, hypoallergenic, and doesn’t trigger immune responses, making it suitable for both external and implantable applications.
In my work with medical product developers, I’ve seen platinum-cured silicone used confidently in catheters, surgical drains, implantable seals, and feeding tubes. These applications require the highest standards of safety and cleanliness, which silicone extrusion meets consistently.
What makes silicone so trusted is its compliance with international standards. Most medical-grade extrusions are tested to meet USP Class VI, ensuring that they are free from cytotoxicity and other biological risks. When materials also comply with ISO 10993 and FDA 21 CFR 177.2600, they qualify for a wide range of Class I and Class II medical devices.
“In the medical field, a material isn’t chosen just for performance—it’s chosen for its ability to do no harm. Silicone excels in both.”
Because of this safety profile, hospitals and OEMs worldwide rely on silicone extrusion for products that come into direct contact with patients—especially in neonatal, respiratory, and surgical care where material reactions must be virtually nonexistent.
Precision and Customization
In medical manufacturing, even the smallest deviation in dimensions can have serious consequences. That’s why tight tolerance control is one of the strongest advantages of silicone extrusion. The process allows for extremely consistent cross-sections—sometimes down to ±0.05 mm—making it ideal for devices that require high accuracy and repeatability.
I’ve worked on projects involving micro-extruded silicone tubing with inner diameters as small as 0.2 mm. These are commonly used in minimally invasive surgical tools, drug infusion catheters, and sensor housings, where precision isn’t a luxury—it’s a necessity.
Silicone extrusion is also incredibly versatile when it comes to design. Manufacturers can create custom dies to produce almost any profile: D-shapes, T-slots, star-shaped lumens, or multi-channel tubing for fluid separation. Engineers can tailor:
- Wall thickness
- Shore hardness (softness)
- Transparency or color coding
- Multi-lumen geometries
This customization gives medical OEMs the ability to innovate with confidence. For example, a developer of a next-gen insulin pump can specify a silicone extrusion with precise flow rate control and integrated sealing features, reducing the number of components in the final device.
“The true value of silicone extrusion lies not only in consistency, but in its flexibility to match exact device designs—down to the micron.”
Whether it’s a simple drainage tube or a sophisticated diagnostic connector, silicone extrusion delivers tailored functionality without compromising on scalability or compliance.
High Purity and Clean Manufacturing
Medical environments demand an uncompromising standard of cleanliness, and silicone extrusion meets that challenge head-on. From material formulation to the final extrusion process, everything is engineered to minimize contamination risks—making it the go-to choice for sensitive clinical applications.
At Kinsoe, we produce platinum-cured silicone tubing in ISO Class 7 and Class 8 cleanrooms, ensuring the absence of dust, microbes, and airborne particulates. This is crucial for components that will be used in IV delivery systems, implantable devices, or open-wound contact products. Without a clean extrusion environment, even the highest-quality silicone could become compromised during production.
Silicone itself is an inherently pure material—free of plasticizers, phthalates, BPA, and other leachable additives. This means there’s no risk of toxic migration into medications, bloodstreams, or tissue. It also ensures stability during sterilization and long-term use.
For manufacturers aiming to meet FDA, USP, or ISO 13485 standards, silicone extrusion provides the required traceability and batch control. Every spool, tube, or profile can be tracked to its original raw material lot, extrusion date, and curing parameters—critical for risk management and product recalls.
“High purity isn’t just a feature. It’s a requirement for life-saving devices—and silicone extrusion is built for it.”
This clean manufacturing capability is particularly valuable in the era of single-use medical devices, where cross-contamination risks must be eliminated. Whether used in blood-contact tubing or diagnostic sampling ports, extruded silicone ensures the safety and sterility modern healthcare demands.
Flexibility and Softness
One of the defining characteristics of medical-grade silicone is its unique balance of softness and structural integrity. In many healthcare applications, devices must conform to the body’s curves without causing irritation, pressure points, or damage to delicate tissue. That’s where silicone extrusion truly shines.
Silicone elastomers can be formulated to very low durometers (softness ratings), making them ideal for direct contact with skin, organs, and mucous membranes. At the same time, the extruded profiles maintain dimensional stability—a feature I’ve found particularly important in applications like peristaltic pump tubing and respiratory interfaces, where both flexibility and shape retention are critical.
Here are a few examples of how this property translates to better medical design:
- Feeding tubes: Soft enough to insert with minimal trauma, yet stiff enough to resist collapse during suction or flow.
- Wearable devices: Such as biosensor patches and infusion systems that require long-term skin contact without discomfort or allergic reaction.
- Orthopedic braces and cushions: Where comfort, pressure distribution, and anti-slip properties are essential.
Unlike thermoplastics that can become brittle, or rubbers that may lose elasticity over time, medical-grade silicone retains its flexibility through aging, heat cycles, and sterilization. This makes it invaluable for both reusable and disposable devices.
“The softer the contact, the safer the interaction—especially in devices used inside the human body.”
From neonatal care to geriatric support, the gentle nature of extruded silicone makes it the material of choice for designers prioritizing patient comfort and protection.
Sterilization Resistance
Sterilization is a non-negotiable step in medical device preparation, and the materials used must endure these processes without degrading or compromising performance. Silicone, especially in extruded form, is exceptionally resilient to all major sterilization methods.
I’ve worked with manufacturers who needed their silicone tubing to undergo multiple sterilization cycles—autoclaving, gamma irradiation, and ethylene oxide (ETO)—and the results were consistently reliable. Unlike plastics that can become brittle or leach chemicals after exposure, medical-grade silicone retains its elasticity, transparency, and mechanical properties.
Let’s look at the three main sterilization methods and how silicone holds up:
- Autoclaving (steam at 121–134°C): Silicone’s thermal stability allows it to withstand repeated cycles without softening or deforming. This is especially valuable in surgical settings where reusability is common.
- Gamma Radiation: Often used for pre-packaged disposable devices. Silicone doesn’t break down or discolor as quickly as many thermoplastics under ionizing radiation.
- ETO Gas: For delicate devices that can’t tolerate high heat, ETO is ideal. Silicone’s permeability and chemical resistance make it a safe choice, with minimal residue retention.
“A great material isn’t just about initial performance—it’s about durability after rigorous sterilization.”
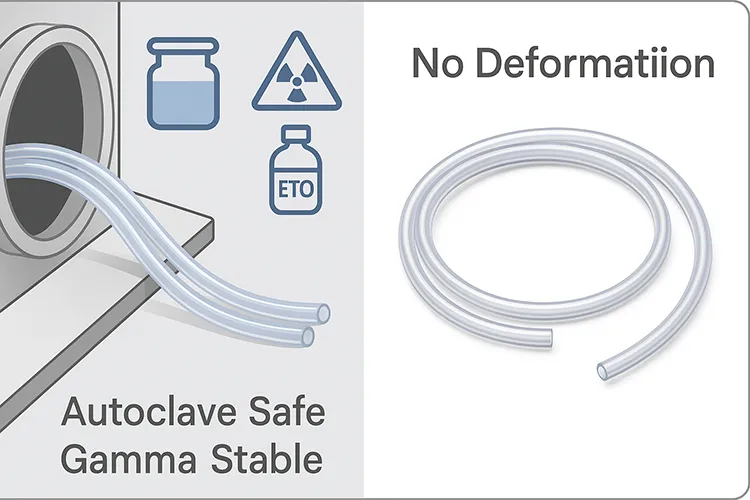
These sterilization-resistant properties make silicone extrusion essential for a wide range of critical devices, such as:
- Catheters
- Suction lines
- Ventilator tubing
- Implant access ports
Whether the goal is single-use sterility or long-term durability, silicone extrusions meet the sterilization demands of modern healthcare without compromise.
Thermal and Chemical Stability
Medical devices are often exposed to extreme environments—whether it’s the heat of sterilization, the cold of storage, or the chemical complexity of medications and bodily fluids. In these situations, silicone’s thermal and chemical stability becomes a key performance advantage.
Extruded silicone can function across an exceptionally wide temperature range, typically from -60°C to +200°C, depending on the formulation. This makes it ideal for both cold-chain applications and high-temperature sterilization. I’ve seen silicone tubing used seamlessly in cryogenic labs and high-temperature surgical sterilizers—without loss of flexibility, structure, or performance.
Chemical resistance is equally important. Medical-grade silicone resists:
- Aqueous solutions, including saline, glucose, and medications
- Body fluids, such as blood, urine, and gastric acid
- Disinfectants and cleaning agents, including alcohols, peroxides, and iodine solutions
This chemical compatibility ensures that no degradation, swelling, or leaching occurs during exposure, which is vital for components like:
- Drug infusion lines
- Dialysis tubing
- Implantable valves and seals
“In a field where failure isn’t an option, materials must remain stable—no matter what they encounter.”
These properties make extruded silicone especially suited to high-reliability, long-term medical applications, where dimensional stability and cleanliness must be maintained despite harsh operational conditions.
Cost Efficiency in High Volumes
While performance and safety are essential, medical device manufacturers must also consider scalability and cost-effectiveness. This is where silicone extrusion delivers significant advantages in high-volume production.
Because extrusion is a continuous process, it allows for long, uninterrupted runs of consistent tubing, cords, or profiles. This reduces downtime, minimizes waste, and lowers the per-unit cost—especially when compared to batch-based processes like molding. For example, I’ve seen OEMs cut their silicone tubing costs by over 30% simply by switching from cut-to-length molding to spool-based extrusion.
This makes silicone extrusion especially well-suited for:
- Disposable medical devices like feeding tubes, suction lines, and drug delivery channels
- Single-use surgical kits that require high-volume, cost-controlled components
- OEM private labeling where tubing is customized but produced at scale
Furthermore, many extrusion lines are automated with inline measurement and defect detection, reducing labor costs and improving first-pass yield. By investing in proper tooling and planning, manufacturers can achieve impressive economies of scale—even when producing custom cross-sections or multi-lumen configurations.
“Silicone extrusion offers the rare combination of medical-grade quality and manufacturing efficiency—critical in today’s healthcare economy.”
Ultimately, when product demand is measured in hundreds of thousands or more, extrusion becomes the most practical and economical method, without sacrificing performance or compliance.
Practical Application
![]()
The real measure of any manufacturing process is how well it performs in actual medical devices—and silicone extrusion is everywhere in clinical practice. From emergency rooms to long-term care facilities, extruded silicone components play a vital role in patient care and medical device functionality.
Here are some of the most common and impactful applications I’ve encountered:
- Surgical Drains: Silicone’s flexibility and biocompatibility make it ideal for passive and active drain systems used post-surgery. These extrusions conform to the patient’s body while resisting kinks and blockages.
- Peristaltic Pump Tubing: In infusion pumps, dialysis machines, and biopharmaceutical equipment, extruded silicone tubing can endure constant compression cycles without fatigue—delivering accurate and consistent flow rates.
- IV Components: Silicone is often used for drip chamber seals, flexible connectors, and access ports. Its clarity and purity ensure no chemical interference during drug delivery.
- Implantable Leads and Seals: In pacemakers and neurostimulators, silicone extrusion provides insulation and environmental protection. Its long-term stability under physiological conditions makes it perfect for permanent implantation.
- Respiratory Masks and Connectors: Soft, hypoallergenic silicone profiles improve patient comfort during non-invasive ventilation (CPAP, BiPAP) or oxygen therapy. They ensure airtight seals without causing skin irritation.
“Every time I walk through a hospital, I see the fingerprints of silicone extrusion—quietly supporting critical care behind the scenes.”
These real-world applications highlight not only the material’s versatility but also the process’s adaptability—enabling manufacturers to meet strict technical demands while still delivering at scale.
Challenges and Considerations
While silicone extrusion offers clear advantages, it’s important to recognize that the process also presents technical and operational challenges—especially in the medical industry, where regulatory compliance and reliability are paramount.
1. Tooling Costs and Lead Time
To achieve precise shapes and tolerances, custom dies must be designed and manufactured. For companies producing small batches or prototypes, this upfront tooling investment can be a hurdle. Complex profiles may also require multi-stage tooling or co-extrusion, further increasing costs and lead time.
“In my experience, custom extrusion projects succeed when the design phase includes both engineering and manufacturing input—up front.”
2. Strict Quality Control Requirements
The margin for error in medical device components is extremely low. Any variation in wall thickness, lumen size, or cure profile can affect device safety and performance. That’s why extrusion operations must include:
- Inline laser measurement systems
- Batch traceability
- Material lot certification
- Full validation documentation (IQ/OQ/PQ)
Even experienced suppliers must maintain FDA-registered or ISO 13485-certified facilities to ensure end-to-end quality assurance.
3. Material Supply Consistency
Medical-grade silicone compounds must meet strict purity standards, and any change in formulation or supplier can trigger a full revalidation. Manufacturers must work with stable, audited material sources and maintain clear documentation for every batch to avoid compliance risks.
“Precision manufacturing doesn’t begin on the production line—it starts with material integrity.”
Despite these challenges, the benefits of silicone extrusion far outweigh the complexities—especially when working with a reliable partner who understands medical device regulations and production nuances.
Conclusion
In the world of medical manufacturing, where lives depend on the performance of every component, silicone extrusion has earned its place as the preferred process. Its unmatched combination of biocompatibility, precision, cleanliness, flexibility, and sterilization resistance makes it ideal for everything from disposable tubing to implantable seals.
As someone deeply involved in the rubber and silicone industry, I’ve seen firsthand how this process transforms innovative ideas into life-saving solutions. Whether it’s micro-extruded catheters for neonatal care or durable connectors for surgical devices, silicone extrusion enables manufacturers to meet the demanding standards of modern medicine—consistently and at scale.
Of course, success depends on more than just the material and process—it requires a skilled partner who understands the regulatory landscape, quality requirements, and technical nuances of medical production.
If you’re developing a new medical device or improving an existing one, I highly recommend working with an experienced silicone extrusion supplier. The right team can help you balance performance, cost, and compliance—without compromise.
References:

